Iso 13485 Medical Device File Template

With the incorporation of clause 4 2 3 on the subject of medical device files in iso 13485 2016 the standard has offered improved value for organisations opting the implementation of standard.
Iso 13485 medical device file template. 3 collection of quality audits. Description of the device. Manage quality throughout the life cycle of a medical device with iso 13485. If you have one to know it should be this one.
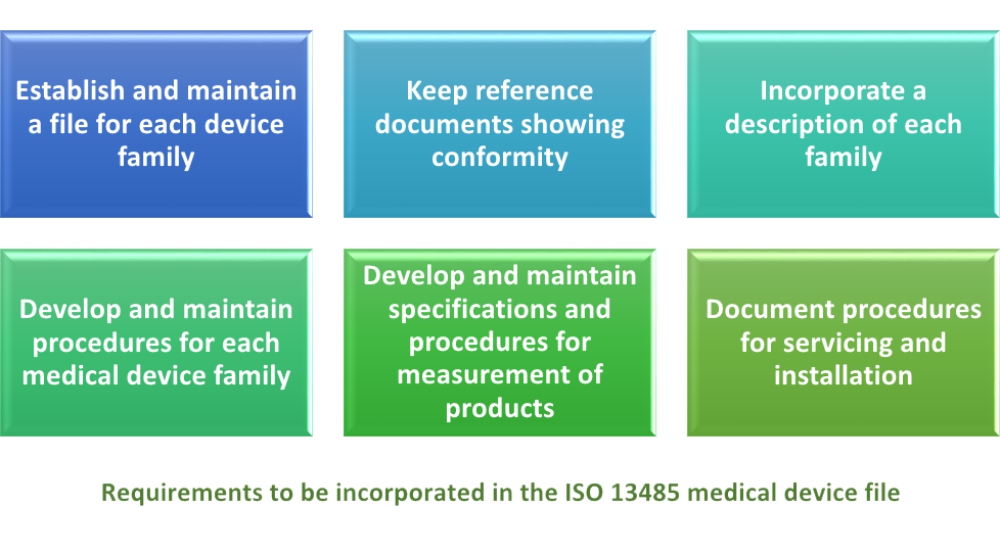
The 2016 version of iso 13485 introduced the medical device file. A quality system and here is why we need iso. Manufacturers and suppliers of medical devices must manage hundreds if not thousands of different medical. Here the standard emphasis on the requirement of establishing and maintaining a medical device file for each medical product or family.
Iso 13485 2016 audit checklists to identify gaps in your organization s qms and prepare for certification. Each of these is very similar in requiring the manufacturer to provide a recipe per medical device type family including the details required to build the medical device. The new edition of iso 13485 contains a sub clause 4 2 3 medical device file under the section documentation requirement. Achieve iso 13485 certification and maintain the quality of medical devices.
The requirements for medical device files in iso 13485 2016 are an endeavor by the iso technical committee tc 210 to create consistent operations for medical device manufacturers and also to make their quality management systems compliant with the rules of various regulatory bodies. Labeling packaging marking instructions for use installation and maintenance instructions device specification. The medical device file should contain the below documents or the reference of the documents. The name of this standard is.
Originally published in 1996 this documentation was the first template quality system for the medical device industry. Now in its fourth edition and with over 3 000 copies sold it has helped thousands of companies to achieve iso 13485 registration and or fda compliance. Medical devices quality management systems requirements for regulatory purposes. This file must provide similar information.
A free brochure with tips for getting started with iso 13485. 1 iso 13485 audit checklist. To be able to sell your medical devices in europe you need 2 things. Caring about health and safety.
2 iso 13485 2016 standard checklist. Similarly iso 13485 2016 requires medical device manufacturers to establish and maintain a mdf per medical device type or medical device family. Elements of medical device files. Uncover how iso standards help doctors treat patients and keep people safe at work at home wherever.